Usually two or more phase 3 trials are conducted. Phase 23 Clinical Trial means a human clinical trial in any country that satisfies the requirements for both a Phase II Clinical Trial and a Phase III Clinical Trial and is designed a to ascertain efficacy and safety of a Licensed Product and b to be sufficient to support the preparation and submission of an NDA for such Licensed Product to a competent regulatory authority.
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
The most reliable study design is a randomized blinded and placebo- or active-controlled trial to verify that the results are beneficial.

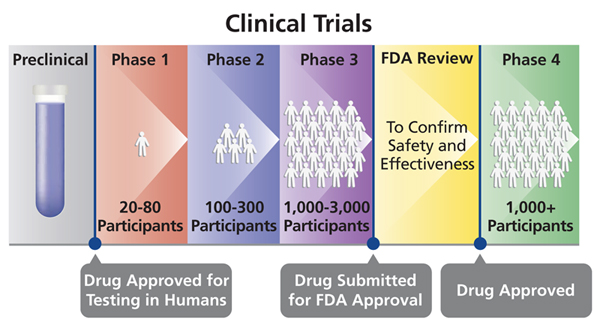
Phase 3 clinical trial definition. Phase 3 is the last phase of testing to be completed before the drugs details and clinical trial results are submitted to the regulatory authorities for. Phase III of a clinical trial usually involves up to 3000 participants who have the condition that the new medication is meant to treat. ResTORbio completes patient enrollment of Phase 3 PROTECTOR 1 trial.
The FDA reviews the results from the clinical trials and other relevant information. A type of intervention model describing a clinical trial in which groups of participants receive two or more interventions in a specific order. Phase III phase III clinical trial - a large clinical trial of a treatment or drug that in phase I and phase II has been shown to be efficacious with tolerable side effects.
Clinical Research While preclinical research answers basic questions about a drugs safety it is not a substitute for studies of ways the drug will interact with the human body. Phase III Clinical Trial Phase III studies are done to study the efficacy of an intervention in large groups of trial participants from several hundred to several thousand by comparing the intervention to other standard or experimental interventions or to non-interventional standard care. Phase 3 clinical trials are designed to test whether the investigative treatment is better than the standard clinical method for the targeted health condition.
One group receives drug A during the initial phase. Researchers design clinical trials to answer specific research questions related to a drug candidate. Clinical trials follow a rigorous series from early small-scale Phase 1 studies to late-stage large scale Phase 3 studies.
Phase 3b Clinical Trial means a human clinical trial of the Licensed Product that a is voluntarily conducted after the filing with the FDA of the NDA for the Licensed Product but prior to obtaining Regulatory Approval of such NDA and b is not required or requested by the FDA as a condition of or in connection with obtaining such Regulatory Approval. The term clinical trials or clinical research refers to studies that are conducted in people. PROTECTOR 1 is the first of two global Phase 3 clinical trials evaluating the potential of RTB101 to improve the immune function of patients aged 65 and older and thereby decrease the incidence of illness associated with respiratory tract infections.
When phase 3 clinical trials or sometimes phase 2 trials show a new drug is more effective and safer than the current standard treatment a New Drug Application NDA is submitted to the Food and Drug Administration FDA for approval. Clinical trials are studies intended to discover or verify the effects of one or more investigational medicines. The regulation of clinical trials aims to ensure that the rights safety and well-being of trial subjects are protected and the results of clinical trials are credible.
For example two-by-two cross-over assignment involves two groups of participants. After successful conclusion of these clinical trials it will receive formal approval from the FDA. In the United States when phase III clinical trials or sometimes phase II trials show a new drug is more effective or safer than the current treatment a new drug application NDA is submitted to the Food and Drug Administration FDA for approval.
The primary endpoints of confirmatory Phase 3 trials should represent directly or through a validated surrogate something that matters to a patient.

Clinical Trial Medicine Britannica

The Biosimilar Approval Process How Different Is It Considerations In Medicine

Phase Iii Randomized Trial Of Mirvetuximab Soravtansine Versus Chemotherapy In Patients With Platinum Resistant Ovarian Cancer Primary Analysis Of Forward I Annals Of Oncology
Analytics And Metrics Help Pinpoint Costs Of Study Startup

Clinical Trial Medicine Britannica

Regulatory Approval Clinical Trial Medical Monitoring Plan Online Clinical Research Courses In India
Clinical Trials Research Governance Unsw Research

Clinical Trials In India Wikipedia

A Strategic Approach To Covid 19 Vaccine R D Science

Increasing Diversity In Clinical Trials Overcoming Critical Barriers Sciencedirect

The Drug Discovery Process Taconic Biosciences
Translational Research Defining The T S Translational Cancer Research Network
The Phases Of Preclinical And Clinical Trials

Artificial Intelligence For Clinical Trial Design Trends In Pharmacological Sciences

Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals

Siponimod Versus Placebo In Secondary Progressive Multiple Sclerosis Expand A Double Blind Randomised Phase 3 Study The Lancet

The Drug Discovery Process Taconic Biosciences

Therapeutic Outcome Of Early Phase Clinical Trials In Multiple Myeloma A Meta Analysis Blood Cancer Journal

